Neurx Diaphragm Pacing System
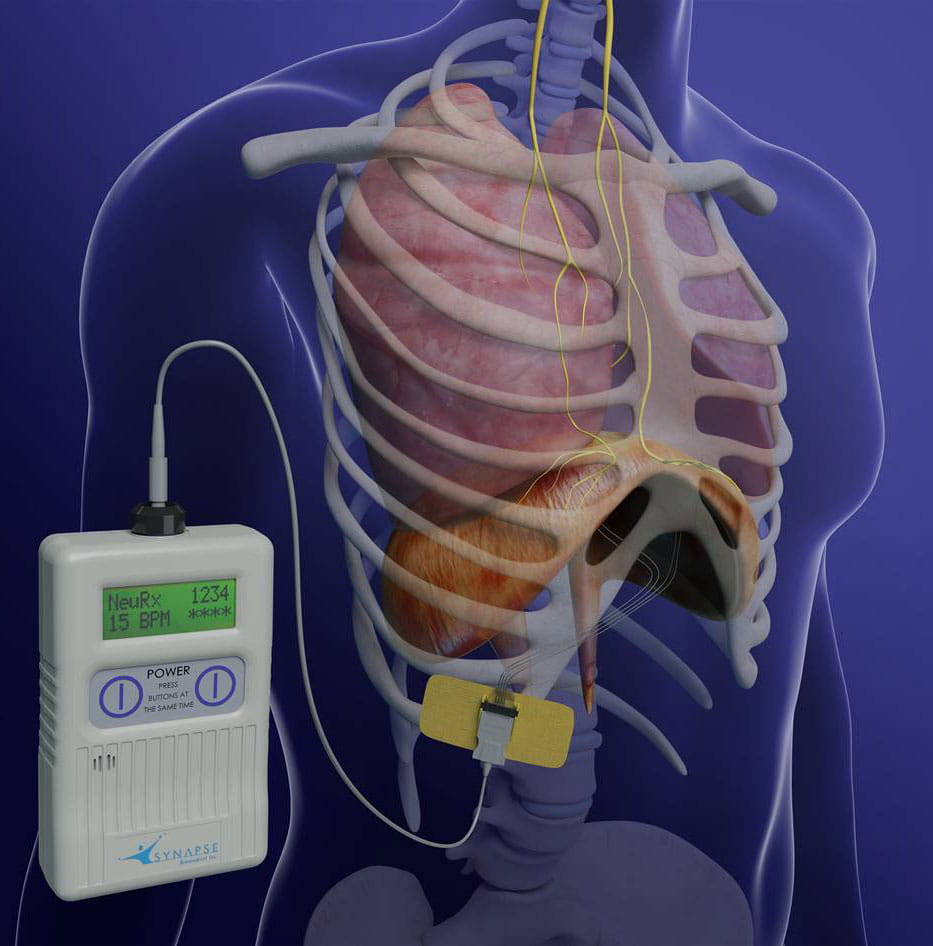
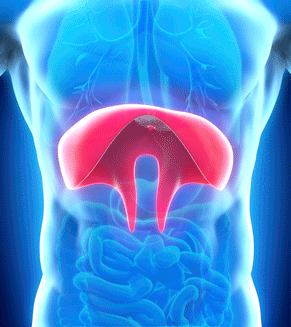
Neurx diaphragm pacing system. NEURX DIAPHRAGM PACING SYSTEM. NEURX DIAPHRAGM PACING SYSTEM. The NeuRx Diaphragm Pacing System DPS provides electrical stimulation of the diaphragm muscles to cause diaphragm contractions to affect air to be taken into the lungs under negative pressure.
Mechanical ventilation is associated with functional limitations such as decreased mobility and. NeuRX Diaphragm Pacing System. This device is indicated for use in amyotrophic lateral sclerosis als patients with a stimulatable diaphragm both right and left portions.
Amyotrophic lateral sclerosis ALS also known as Lou Gehrigs disease is a progressive neurodegenerative disease of nerve cells affecting the brains ability to control muscle movement and may lead to paralysis and death. The NeuRx System is particularly important because previous ventilation methods have been shown to cause atrophy and accelerate respiratory compromise. The ProprietaryTradeBrand name of the medical device as used in device labeling or in the catalog.
Sabrina Drexel discusses her experience with the NeuRx Diaphragm Pacing System. In 3-4 years the diaphragm pacing system can pay for itself with the cost savings. Diaphragm Pacing System Gets FDA Nod for ALS.
NeuRx Diaphragm Pacing System. NEURX DIAPHRAGM PACING SYSTEM. The NeuRx Diaphragm Pacer with its minimally invasive approach is both scientifically and clinically proven to preserve muscle strength and maintain vital capacity.
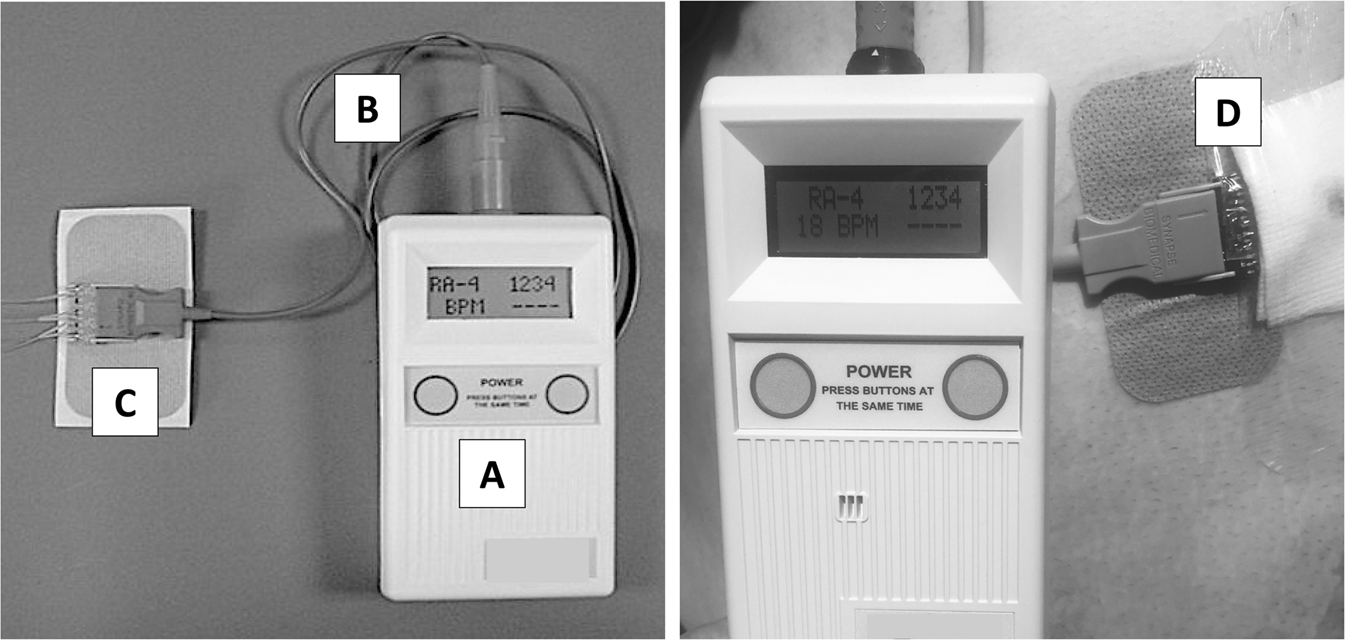
Food and Drug Administration FDA such as the NeuRx DPS RA4 Respiratory Stimulation System Synapse Biomedical Inc Oberlin OH which is an implantable electrical device that stimulates the diaphragm. Madonna Rehabilitation Hospital is among a handful of institutions nationwide and the only Nebraska rehabilitation hospital that. CLEVELAND June 18 PRNewswire -- Synapse Biomedical Inc.
Approval for the neurx dpstm diaphragm pacing system. Thats a savings of up to 20000 per year.
Since DPS has not been rigorously evaluated in ALS populations a clinical trial has been designed to scientifically determine the effectiveness on improvement of diaphragm function and survival in ALS patients.
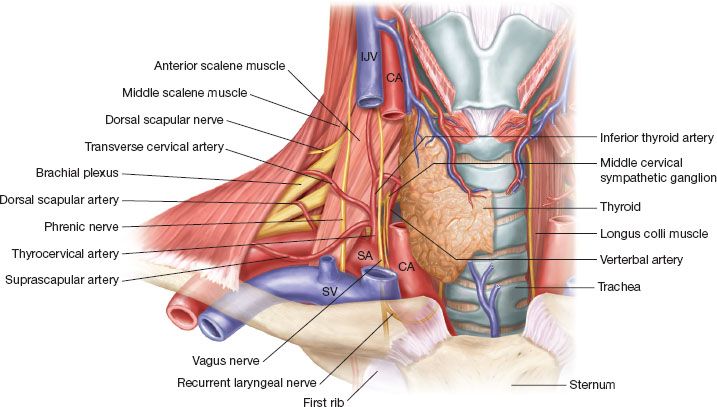
Food and Drug Administration FDA has approved the NeuRx Diaphragm Pacing System DPS for spinal cord-injured patients who are dependent on. The NeuRx System is particularly important because previous ventilation methods have been shown to cause atrophy and accelerate respiratory compromise. September 30 2011 The US Food and Drug Administration FDA has granted approval for the. Approval for the neurx dpstm diaphragm pacing system. On b 6 2011 the patient. Amyotrophic lateral sclerosis ALS also known as Lou Gehrigs disease is a progressive neurodegenerative disease of nerve cells affecting the brains ability to control muscle movement and may lead to paralysis and death. Thats a savings of up to 20000 per year. The DPS system consists of four implantable Permaloc intramuscular electrodes inserted into the muscles of the diaphragm and a reference electrode which is placed under the skin. The NeuRx DPS device implanted during a minimally invasive surgery helps individuals breathe easier by conditioning their diaphragm muscle through electrical stimulation.
This device is indicated for use in amyotrophic lateral sclerosis als patients with a stimulatable diaphragm both right and left portions. TransAeris builds on the success of another Synapse Biomedical technology the NeuRx Diaphragm Pacing Stimulation NeuRx DPS System which has been FDA and CE Mark approved since 2008 for people with Spinal Cord Injury SCI and has successfully reduced or eliminated the need for mechanical ventilation. Commercial Distribution End Date. Thats a savings of up to 20000 per year. NeuRx Diaphragm Pacing System for ALS. September 30 2011 The US Food and Drug Administration FDA has granted approval for the. NEURX DIAPHRAGM PACING SYSTEM.
































Post a Comment for "Neurx Diaphragm Pacing System"